What is Nabota?
Nabota is a powerful botulinum toxin type A product. It is derived from Clostridium botulinum, a naturally occurring bacterium. Known for its effectiveness, Nabota is used in both medical and cosmetic fields.
Benefits of Nabota
Medical Applications
- Upper Limb Spasticity: Nabota is widely used to treat upper limb spasticity. Patients injected with Nabota experience significant improvement in muscle control.
- Chronic Migraine: Sufferers of chronic migraine find relief through Nabota. A single injection will significantly decrease the frequency and intensity of migraines.
- Urinary Incontinence: Nabota botulinum toxin helps manage urinary incontinence. Its application in the neuromuscular junction aids in controlling bladder muscles.
- Spinal Cord Injury: Nabota provides therapeutic effects for spinal cord injury patients, reducing muscle stiffness and improving mobility.
Cosmetic Uses
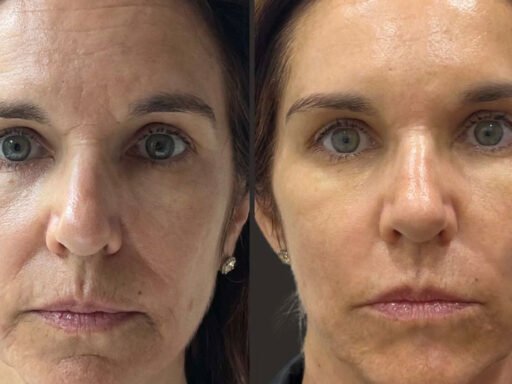
- Eliminating Wrinkles: Nabota is effective in reducing forehead lines, glabellar lines, and other facial lines. Its botulinum neurotoxin type A targets specific muscles, smoothing out wrinkles.
- Focal Hyperhidrosis: Those suffering from excessive sweating (focal hyperhidrosis) may benefit from Nabota. It significantly reduces sweat production.
Clinical Trials and Studies
Randomized Controlled Trials
Multiple randomized controlled trials highlight the effectiveness of Nabota. One study focused on stroke patients with upper limb spasticity. Results showed a significant improvement in muscle control post-injection. Another clinical trial involved adults with chronic migraine. Nabota injections led to a noticeable decrease in migraine episodes.
Safety and Adverse Reactions
- Adverse Events Reported: Clinical trials have reported some adverse events. Common issues include muscle weakness and temporary discomfort at injection sites.
- Serious Adverse Events: Serious adverse events are rare but may include significant muscle weakness and other complications.
- Safety Evaluation: Continuous evaluations and formal analyses ensure that Nabota maintains a high safety profile. Regular monitoring of vital signs and physical examination of patients help detect potential risks early.
Mechanism of Action
Nabota works by blocking nerve signals to muscles. The temporary treatment reduces muscle activity, which helps manage conditions like upper limb spasticity and chronic migraines. The botulinum toxin type A in Nabota is a highly toxic protein, making it effective in small doses.
Application Process
- Injection Sites: Nabota is injected directly into the target muscle. Common injection sites include the forehead, upper limb, and bladder muscles.
- Dosage and Units: Nabota 100 units is a common dosage for treating various conditions. The precise amount depends on the specific needs and conditions of the patient.
- Physical Therapy: Physical therapy often accompanies Nabota injections. Combining both treatments leads to better outcomes in rehabilitation medicine.
Considerations for Use
Patient Selection
Careful patient selection is crucial. Factors like baseline characteristics and previous injections influence the effectiveness of Nabota. Inclusion criteria for clinical trials usually involve patients with specific conditions like upper limb spasticity or chronic migraine.
Statistical Analysis
Data from clinical trials undergo rigorous statistical analysis. Techniques like the Wilcoxon signed rank test ensure the reliability of results.
Potential Risks and Adverse Reactions
- Adverse Drug Reactions: Adverse drug reactions include localized pain and swelling. Some patients might experience muscle weakness and temporary discomfort.
- Muscle Weakness: Temporary muscle weakness may occur in injected muscles. However, it usually resolves within a few days.
- Serious Adverse Events: Though rare, serious adverse events like myocardial infarction and significant muscular weakness require immediate medical attention.
Final Thoughts
Nabota stands out as a versatile and effective botulinum toxin type A product. From treating upper limb spasticity and chronic migraines to eliminating wrinkles, its applications are vast. Clinical trials and safety evaluations affirm its reliability, making Nabota a valuable tool in both medical and cosmetic fields. Always consider the potential risks and consult a healthcare professional before opting for Nabota treatments.
Notably, Nabota’s applications have shown significant improvement in patients, with statistical analyses confirming its efficacy across various muscle groups. With continuous formal analysis and open access articles distributed, the medical community remains informed about its benefits and potential risks. Thus, Nabota remains a critical option for those seeking therapeutic and cosmetic solutions.
Frequently Asked Questions
Is Nabota better than Botox?
Both Nabota and Botox are effective, but individual results may vary.
Is Nabota approved by the FDA?
Yes, Nabota is approved by the FDA.
What is Nabota Botox?
Nabota Botox is a botulinum toxin type A used for medical and cosmetic purposes.
Is Nabota the same as Jeuveau?
Nabota and Jeuveau are similar but marketed under different names in various regions.